Fabricius, 1801
 |
| Fig. 1: Cicindela aurulenta (Fabricius 1801), Female. Image by: Darren Yeo |
Table of Contents
Overview
Cicindela aurulenta is a small yet highly conspicuous tiger beetle (Cicindelinae) that inhabits a wide range of sandy habitats. It is a common species in Singapore and Southeast Asia, and may be found near shorelines, mangrove fragments and forest trails (1). Its distinct appearance and splendid colouration make it highly charismatic and recognizable. Tiger beetles play an important role in the ecosystem as generalist predators and have been reported as effective biological pest-control agents.
Southeast Asian tiger beetles are however, not as well studied or characterized as their more temperate counterparts, despite the Indo-Malayas having the highest tiger beetle diversity (2). Their larvae are even more poorly understood (3). C. aurulenta, as will be shown here, is particularly amenable to rearing and breeding in laboratory conditions with minimal effort, thereby making it an excellent candidate species for further studies in the Indo-Malayas.
 |
| Fig. 2: Image by Thomas Brown. Creative Commons Attribution 2.0 Generic license. |
Distribution
Locality
C. aurulenta was first described in Sumatra but has a rather large range in the Indo-Malayas. C. aurulenta s. str. and its various subspecies have been identified in the following areas:
Habitat
C. aurulenta can be found in a wide range of sandy habitats (1), including riverbars, forest trails, sand dunes near shorelines and mangroves. The Singaporean specimens were collected in sand banks near mangrove fragments.
Biology
Diet
Tiger beetles (Cicindelinae) are primarily generalist predators that feed on any invertebrates they manage to catch with their powerful mandibles. However, they have been known to feed on fruits (4) and scavenge even on dead vertebrates. They actively forage for food, spotting prey with their keen eyesight and quickly running them down. Tiger beetles have been known to run at speeds up to 1.2 miles per hour, which is extremely impressive given their small size. Research has shown that tiger beetles pursue their prey in a stop-and-go discontinuous manner as the speed of their movement confounds the continuous detection of prey (5). After capture, prey is dismembered by their powerful mandibles and digestive juices from the midgut are injected while chewing. The dissolved fluid is then sucked up and ingested (6).
C. aurulenta adults are likewise ferocious predators and are able to tackle prey nearly twice their size. However, they seem to exhibit a size preference, where prey of 2-4 mm was rejected and prey of 5-7 mm was preferred to prey larger than 2 cm. Adults have been shown to recognize prey by familiarity of size, shape and location (7). As such, they should ideally be fed similar prey to that of their native environment. C. aurulenta adults were able to survive on a diet of houseflies (Musca domestica) and mealworms (Tenebrio molitor larvae), although they much preferred the former. The adults may go over two weeks without food, but this compromises their oviposition rates.
C. aurulenta larvae are also predatorial, but employ a different hunting strategy. The larvae lie in wait in vertical burrows and ambush potential prey they happen to encounter. Small enough prey are grasped by the larva’s large mandibles and dragged into the burrow for consumption. The hooks on their dorsal side anchor them to the burrow walls while they subdue larger prey. The visual perception of tiger beetle larvae is complex enough to discriminate moving targets and background (8). First/second-instar larvae were able to subsist on small termite workers, which are easily obtained and kept alive.
Mating Behaviour and Oviposition
 |
| Fig. 3: Mating pair of Cicindela aurulenta, with the male's mandibles around the female's coupling sulcus. Image taken by and used with permission from Nicky Bay. |
If the mounting is successful, the male grabs the female’s thoracic sulcus with his mandibles and adhesive pads on his prothoraic tarsi, which enables him to hold on to the female during copulation. The “coupling sulcus” is a groove in the female’s thorax that is complementary to the mandible shape of males of the same species. This is believed to be a selection mechanism against interspecific breeding (10). During copulation, C. aurulenta males will occasionally hold their metathoraic legs in the air.
C. aurulenta, like most other tiger beetles, exhibit a form of mate guarding. After copulation, the male will remain mounted upon the female’s back for an extended period of time, before eventually being thrown off. The same male will attempt to procure multiple copulations if given the opportunity.
Once the females have accumulated sufficient resources, they will dig a chamber in sandy substrate with their abdomen and lay a single egg in each chamber. On hatching, the beetle larvae will enlarge the chamber into a vertical burrow, the depth of which depends on the instar, species, season and substrate (8). The amount of eggs laid is constrained by the amount of food the female is able to obtain (11).
Ontogeny
C. aurulenta larvae were observed to require about 2 weeks for development from oviposition to eclosure of first instar larvae from the egg, in tropical climates. Tiger beetles generally require 9-29 days for this stage of development, depending on species and temperature (8).
Larvae will undergo three instars before pupation, which may take 1-4 years, although 2 years is the most common natural time period (8). However, some species that have been extremely well-fed under laboratory conditions may develop from egg to adult in 60 days (11). The C. aurulenta larvae kept for observation have not yet been observed to moult, but this is difficult to ascertain as the larvae are elusive and hardly ever venture out of their burrows.
Before pupation, tiger beetle larvae will plug the entrance of their burrows and dig a pupation chamber (8). Ecdysisinto pupal form lasts a few minutes and time taken until emergence can vary from 18-30 days. Adults will emerge from the substrate about 3 days after pupal ecdysis.
Predation & Parasitism
 |
| Fig. 4: Tiphia femorata (Tiphiidae), a major parasite of beetle larvae. Image by: James Lindsey. Creative Commons Attribution-Share Alike 2.5 Generic license. |
Another major parasitoid of tiger beetles are bombyliid flies of the genus Anthrax (13). The female fly will lay her eggs into the tunnel opening of the beetle larvae, where the eggs will tumble into. The larva ecloses, attaches itself onto the beetle’s thorax and consumes the beetle larva only when it begins pupation.
 |
| Fig. 5: Anthrax sp. flies (Bombyliidae) are another group of beetle larvae parasites. Image by: ©entomart. |
Defensive Adaptations
 |
| Fig. 6: Cicindela aurulenta's iridescent colouring and maculation may serve defensive purposes. Image by: Darren Yeo |
C. aurulenta sports an iridescent or metallic colouration, with six large visible spots. Iridescent colouration reflect a narrow range of light wavelengths and hence may change colour dramatically at different viewing angles, as well as darken in low light conditions, thus possibly serving anti-predatory functions (14). This iridescence may also serve as a flash colouration, where a dramatic change in colouration while the beetle takes flight may startle predators enough for the beetle to make its escape.
The spots on the dorsal side of its elytra may be parasemetic colouration, serving as eyespots or deflectors that either ward off predators or deflect attention from more critical parts of the body (15).
More hypothetical reasons for C. aurulenta’s unique colouration may be aposematic colouration or Müllerian mimicry, where bright warning colours are used as signals of toxicity or merely as a copy of a similarly patterned aposemetic species. However, no poisonous tiger beetles have been recorded and given its large range, it is unlikely that C. aurulenta would be sympatric with an aposemetic lookalike. More research is necessary to determine the function of C. aurulenta’s colouration.
Tiger beetle larvae have few defensive mechanisms, relying mainly on their burrows for protection. They will either drop to the base of their burrows or may leave their burrows and flee to another location, where another burrow will be excavated.
Other Adaptive Behaviour
 |
| Fig. 7: Cicindela aurulenta exhibiting stilting behaviour. Image taken by and used with permission from Nicky Bay. |
Conversely, when temperature falls to below comfortable levels, tiger beetles have been observed to bask with their sterna against warm substrates and thereby gain heat conductively (8). C. aurulenta, being a tropical species, prefers higher temperatures and will become sluggish in air conditioned areas.
Tiger beetle larvae have been known to plug their burrows and become inactive during hot dry seasons (8). In extreme conditions of desiccation and flooding, they may also relocate their burrow. Some species of tiger beetle larvae also construct turrets above their burrows to thermoregulate at lower temperatures (16).
C. aurulenta larvae have not been observed to display such turrent-building behaviour, although the burrow is actively and meticulously maintained by the larva; if the burrow entrance caves in or is covered, the larva will dig a new entrance or remove any debris from it. As the burrows of C. aurulenta are on seashores and near mangrove clusters, they risk occasional inundation during high tides. It has been shown however, that species occupying such habitats have an unusually high resistance to anoxic conditions, either by depressing metabolism (17) or possibly having sufficient oxygen in their burrows (9).
Significance
Ecological & Economic Potential
Tiger beetles, being voracious generalist predators, are believed to be potential biological controls for pest species. Pest organisms reported to have been brought under control by tiger beetles include a variety of paddy pests from India (18), mosquito larvae in West Africa (19), mole crickets from the southeastern United States (8), twolined spittlebugs and fall armyworms (20), etc.. Conversely, some studies have also suggested tiger beetles to be ineffective biocontrol agents (21). It is thus difficult to make generalizations and more local studies are necessary to assess impact on pest species. The ecological role of C. aurulenta in its native habitat has not been assessed.
Conservation
 |
| Fig. 8: The eye-catching colouration of most tiger beetles puts them at risk from over-collecting. Image taken by and used with permission from Nicky Bay. |
C. aurulenta has a wide range and is hence unlikely to face global extinction. The Singaporean population though, could be threatened locally via habitat loss, seeing how mangrove fragments and natural shorelines are not particularly prioritized in Singapore’s changing landscape and ever-increasing demands for space. Additionally, with natural habitats becoming more fragmented and coming into closer proximity with urban areas, these tiger beetles, being attracted to night-lights, face another source of potential risk. C. aurulenta, with its charismatic appearance and colouration, could be a potential flagship species for habitat conservation.
Tiger beetles have been proposed as good bioindicator taxa for conservation research (23), owing to their well known taxonomy, well understood biology and natural history, easily surveyed populations, broad geographic range, degree of habitat specialization and potential economic importance (24). More recent studies have also reported the use of tiger beetles as indicator species for habitat degradation and trends in conservation biology (25) (26). C. aurulenta and other native cicindelids could potentially be important bioindicator taxa for Singapore’s mangrove fragments.
Morphology and Identification
General Anatomy and Characteristics
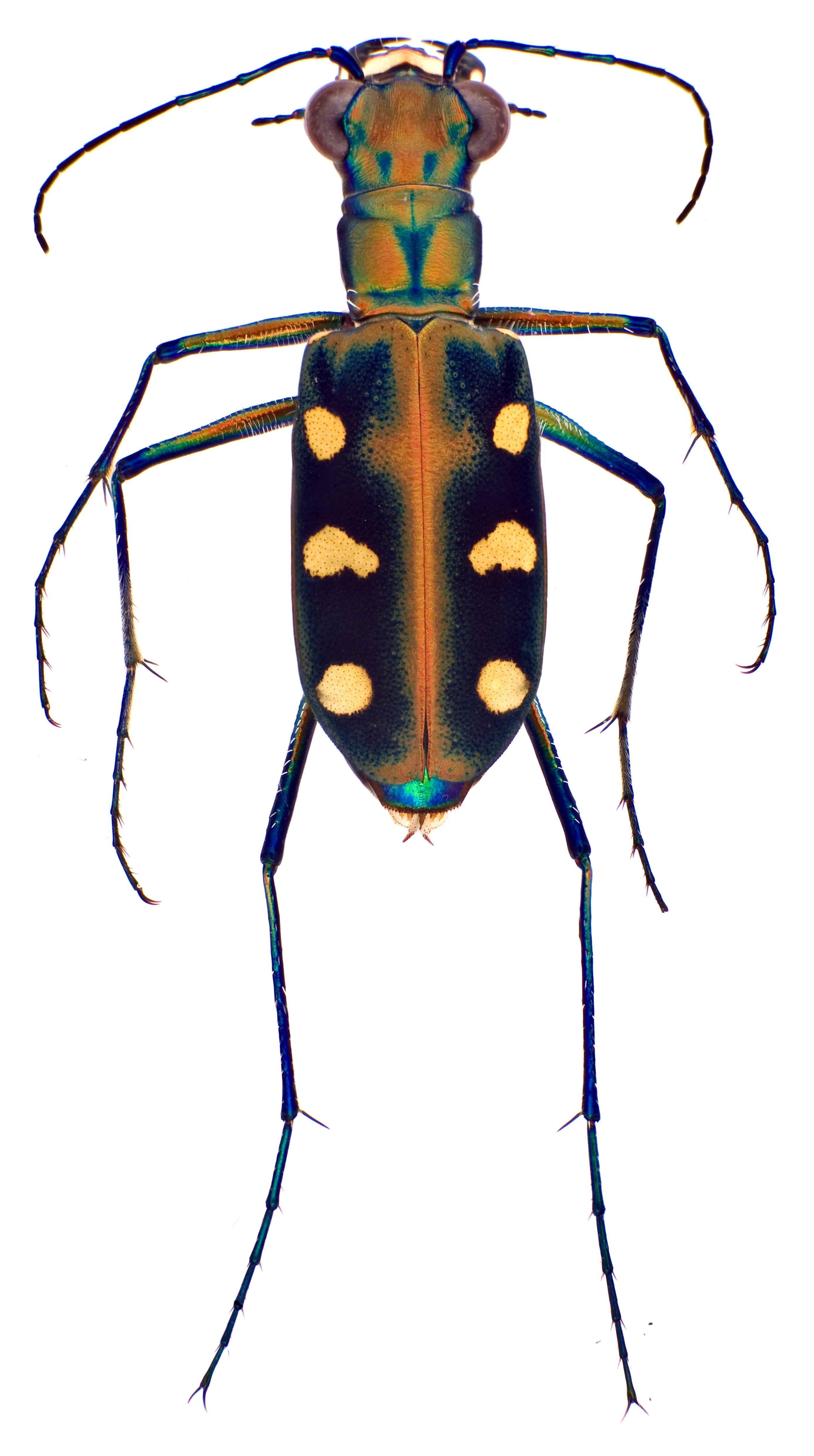
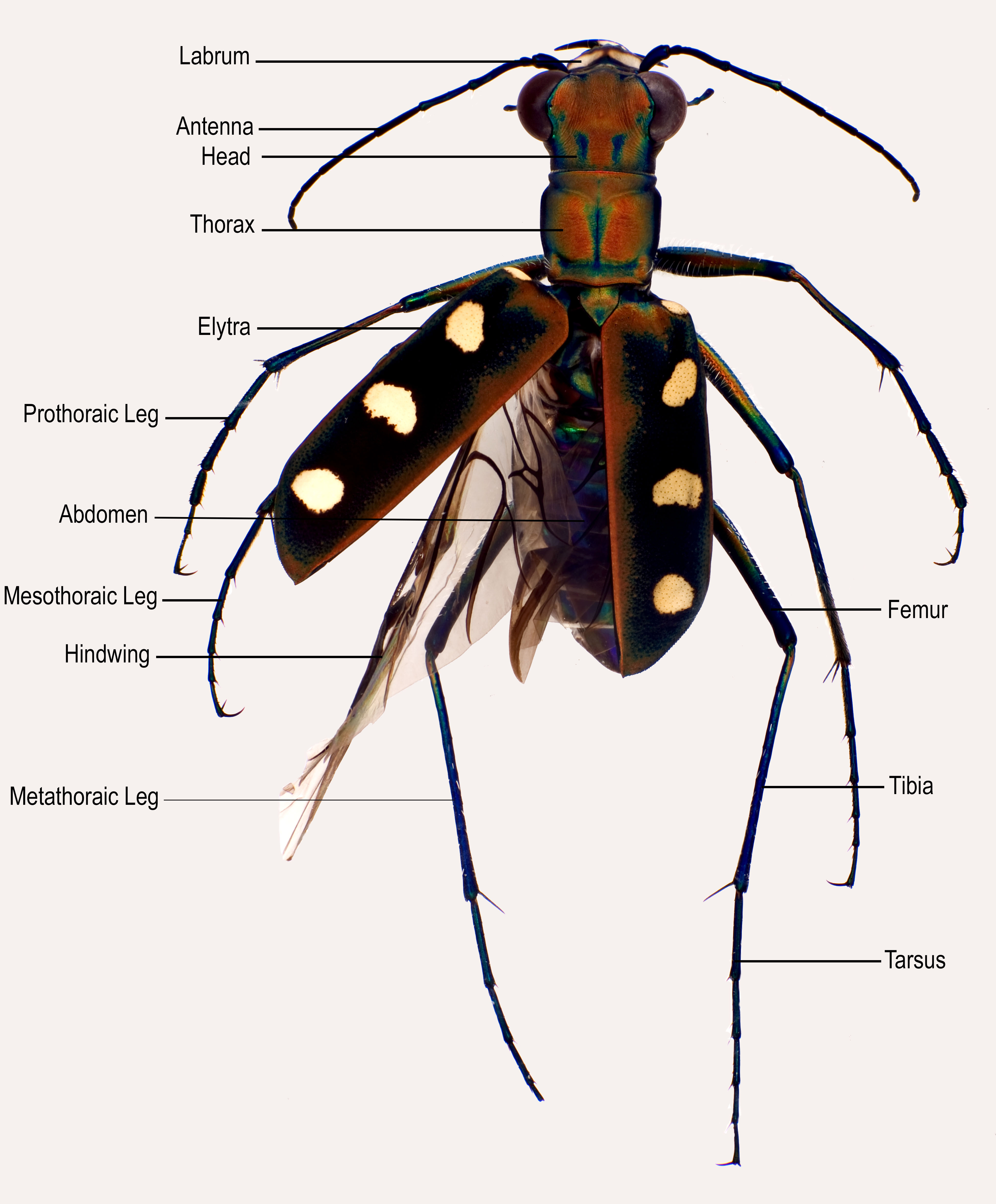 |
| Fig. 9: Cicindela aurulenta adult, dorsal view, showing general beetle anatomy. Image by Darren Yeo. |
 |
| Fig. 10: Cicindela aurulenta adult, fronta view, showing tiger beetle mouthparts. Image by Darren Yeo. |
 |
| Fig. 11: Cicindela aurulenta larva, dorsal view, showing general beetle larva anatomy. Image by Darren Yeo. |
Larval Images
The larvae of C. aurulenta has not been formally described nor imaged. I have managed to rear first/second-instar larvae thus far and have provided whole images of them below, as well as particular key characteristics generally used for larval identification. Any expertise on tiger beetle larvae description and identification is extremely welcome.
 |
| Fig. 12: Cicindela aurulenta first instar larvae, dorsal view. Image by: Darren Yeo |
 |
| Fig. 13: Cicindela aurulenta first instar larvae, ventral view. Image by: Darren Yeo |
 |
| Fig. 14: Cicindela aurulenta first instar larvae, lateral view. Image by: Darren Yeo |
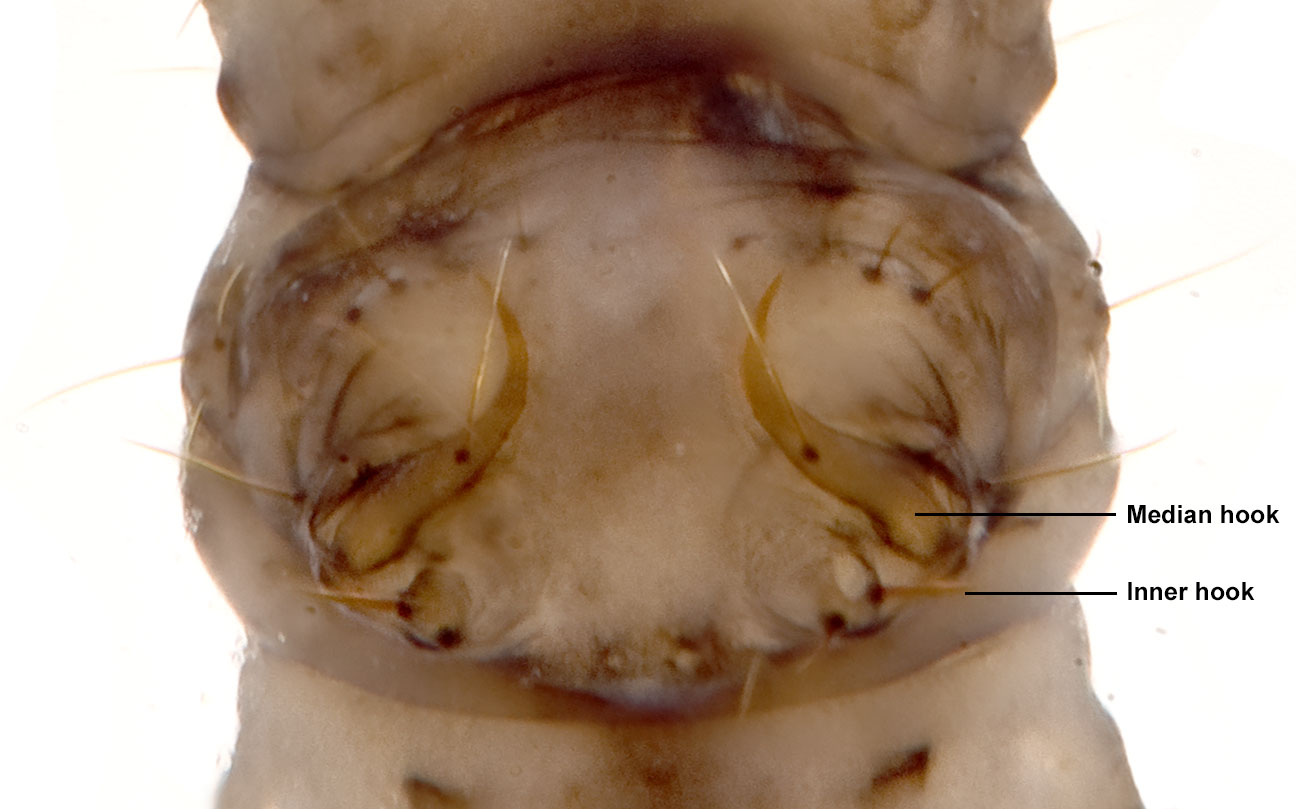 |
| Fig. 15: Hooks and setae on 5th abdominal tergite, dorsal view. Image by: Darren Yeo |
 |
| Fig. 16: Pronotum and associated setae, dorsal view. Image by: Darren Yeo |
Adult Description
The following identifying features have been adapted from "The Naturalist's Library" (27), with reference to C. aurulenta s. str. as first described in "Systema Eleutheratorum" (28). No identification keys including this species have yet been devised.
| Feature |
Property |
Image |
||
|---|---|---|---|---|
| Anterior-posterior length |
|
|||
| Labrum |
|
|
||
| Mandibles |
|
|
||
| Maxillary palps |
|
|
||
| Antennae |
|
|
||
| Head |
|
|
||
| Thorax |
|
|
||
| Elytra |
|
|
||
| Body and Legs |
|
|
Sexual Dimorphism
Tiger beetles exhibit several sexually dimorphic traits, including body size, mandibular length and shape, labrum shape, elytra shape etc. (29). The most reliable ways of differentiating between the two sexes seem to be 1) a groove in the female's thorax known as the coupling sulcus (Fig. 25) and 2) setal pads on the tarsi of the prothoraic legs in males (Fig. 26). C. aurulenta also exhibits distinct differences in labrum pattern (Fig. 27). These characters are further elaborated in the table below.
| Trait |
Male |
Female |
||||
|---|---|---|---|---|---|---|
| Coupling sulcus |
|
|
||||
| Setal Pads |
|
|
||||
| Labrum |
|
|
Taxonomy and Systematics
Synonym
Cicindela aurantiaca (Fleutiaux, 1893)Only one synonym exists for C. aurulenta.
Type Information
The Zoological Museum - University of Copenhagen (ZMUC) houses the lectotype of C. aurulenta (1 specimen) (30).
Subspecies
Cicindela aurulenta aurulenta (Fabricius 1801)Cicindela aurulenta flavomaculata (Chevrolat 1845)Cicindela aurulenta juxtata (Acciavatti & Pearson 1989)Cicindela aurulenta virgula (Fleutiaux 1893)
Tiger beetles typically have many subspecies, due to their many colour variants (9) and geographical distributions (8), resulting in confusion below the species level. Cassola (1) describes the subspecies of C. aurulenta in the following excerpt:
“This common species was found in a variety of habitats, ranging from riverbars to forest trails. The Bornean specimens belong to the typonominal subspecies, which occurs in Malaysia and Indonesia, while from India eastwards to southern China the ssp. juxtata Acciavatti & Pearson 1989 occurs (Acciavatti & Pearson 1989).”
The subspecies of C. aurulenta thus seem to have varying geographical ranges. The Singaporean subspecies is thus most likely C. aurulenta s. str. as the species overlap between Singapore and Malaysia is rather substantial.
Classification
The Taxo-navigation system below is provided by and referenced to UniProt Taxonomy. This reflects the classification of Cicindela aurulenta above the species level.
› Metazoa
Phylogeny
The tiger beetle phylogeny is still contentious and is continuously being reassessed and stabilized with the introduction of molecular methods and the discovery of new species. Owing to the large number of species within this group and their broad geographical range, no proper worldwide phylogenetic study has been done since 1926 (31), where less than half of the current number of species has been discovered and molecular data was unavailable. Additionally, such phylogenetic studies are still lacking in the Indo-Malayas.
The Cicindela clade itself is currently accepted as being monophyletic (32), but relationships above and below the species level are still contentious. The two main areas of contention are: 1) the placement of the tiger beetle clade and 2) the monophyly of the subgenera in Cicindela.
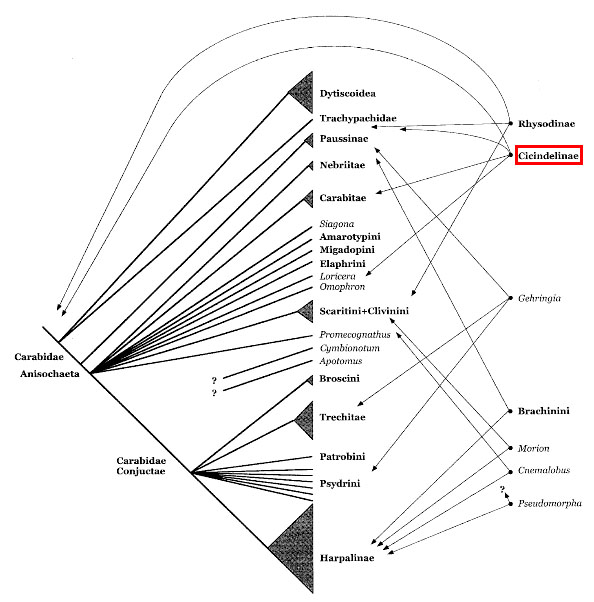 |
| Fig. 28: Relationships of carabid beetles based solely on morphology. The taxa on the right are unresolved and the arrows show proposed placements. Cicindelinae is highlighted in red, illustrating the difficulty of its placement (Maddison et al., 1999). Obtained and accredited under fair use. |
Tiger beetles have been regarded as various monophyletic clades: Cicindelitae within the Carabidae (33), as Cicindelidae apart from Carabidae (34) and as Cicindelini (35) within Cicindelinae. The most recent phylogenetic analysis places tiger beetles within the carabid phylogeny (36), although the placement is again contentious; some have placed the group at a basal position or higher in the tree (32). Currently, the most widespread tiger beetle classification nests the group within Carabidae, subfamily Cicindelinae, tribe Cicindelini (eg. NCBI Genbank, Boldsystems, Encyclopedia of Life, UniProt).
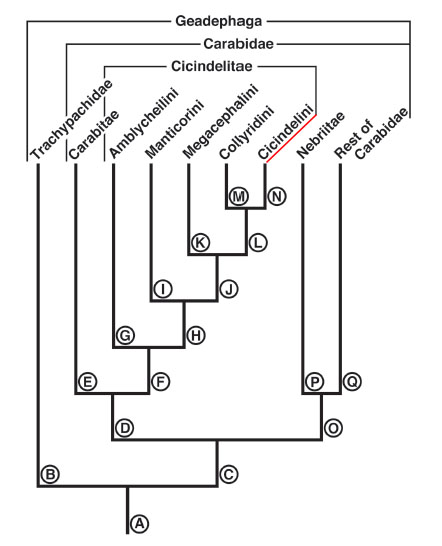 |
| Fig. 29: The most current consensus places the Cicindelini within the Carabidae. Tree obtained from a study by Ball et al. (2011) on tiger beetle mandible and labrum evolution. The letters correspond to the lineages studied. Obtained and accredited under fair use. |
Another part of the tree that is still being reviewed is a consequence of the splitting of the genus Cicindela by Rivalier (37) into some 55 subgenera based mainly on male genitalia. However, a number of the subgenera proposed were not monophyletic (38) and hence several studies have sought to resolve this division. This is still an ongoing effort as several groups remain hard to place despite the use of molecular methods (39). C. aurulenta now falls under the subgenus Cosmodela.
A wide variety of characters have been used to construct these phylogenetic hypotheses. Morphological characters include genitalia (37), immunological responses (40), eye structure (41), elytral pattern (42), microsculpture (43), metathoraic wing structures (44), tibial antenna cleaners (45) as well as mandible and labrum structure (46). More recently, molecular analyses have been used to reassess phylogenies, using chromosome number (47), mtDNA (48), rDNA (32) and exon and intron sequences (39).
The bulk of these phylogenetic studies still suffer from insufficient taxon sampling and diagnostic characters. With heightened sampling of tropical and subtropical species and the increasing feasibility of next generation sequencing, the afore-mentioned issues in tiger beetle phylogeny may finally be resolved. C. aurulenta has not been included in any formal phylogenetic study.
Karyology
The karyology of tiger beetles is highly interesting as they are one of the few organisms with two to four multiple X sex chromosomes (9). These sex chromosomes are unique among insects as they do not form chiasmata and hence do not recombine during meiosis. Instead, these chromosomes do not align in parallel during meiosis but link together at their ends, forming a circle. In the genus Cicindela, all but two species have multiple male sex chromosomes (XnY) (8). Most species of Cicindela have about 14-20 autosomes.
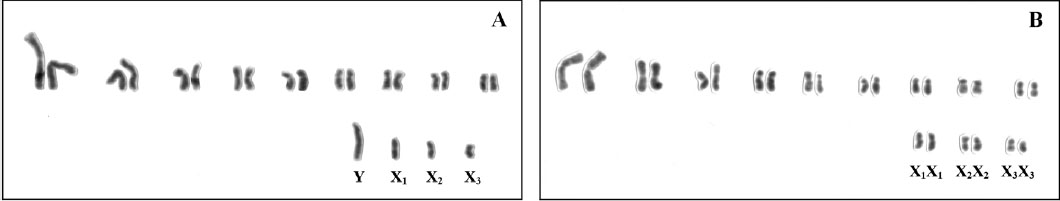 |
| Fig. 30: Mitotic and meiotic chromosomes of Cicindela aurulenta male (left) and female (right), exhibiting multiple X chromosomes (Proença et al., 2004). Obtained and accredited under fair use. |
C. aurulenta was found to have the following karyotype: 2n = 18 + X1X2X3Y/X1X1X2X2X3X3, thereby having 18 autosomes and one set of sex chromosomes with 3 X chromosomes (49). Proença’s study also identified rDNA localization in the X chromosomes, which suggests that these heterosomes are functionally active and also necessary in females. The unusual karyology of cicindelids is still the object of much research and may reveal evolutionary relationships within this speciose group.
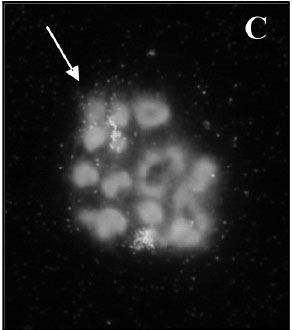 |
| Fig. 31: Fluorescent in situ hybridization with 18S probe of Cicindela aurulenta (male) chromosomes. There are two signals in the sex complex and two in an autosomal bivalent (Proença et al., 2004). Obtained and accredited under fair use. |
Rearing & Breeding
C. aurulenta can be very easily bred in laboratory conditions. Adult individuals are best obtained undamaged by sweep-netting and are easily sexed via the sexually dimorphic characters described above. Larvae have been reported to be easily sampled using a method known as “fishing”, where a piece of grass is dangled at the mouth of the burrow and pulled up when the larvae grab onto it with their mandibles (50). This method however, takes some skill and seemed ineffective with first instar C. aurulenta larvae. Substrate from their native habitat should also be obtained to provide gravid females with oviposition sites.
 |
| Fig. 32: Sandy substrate from the beetle's native habitat should be used when rearing them. Image by: Darren Yeo |
Adults will mate often, but females will only lay if there is sufficient food and a proper substrate for oviposition. To maximize number of offspring and developmental time, both adults and larvae should be fed as much as possible. Adults and larvae may be kept in the same container as C. aurulenta has not been observed to cannibalize offspring, but they are best kept separately. One should also be wary of the introduction of parasitoids when keeping tiger beetle larvae as they are able to easily decimate cultures.
| Fig. 33: A simple terrarium used to rear Cicindela aurulenta adult and larvae. Image by: Darren Yeo |
References
1) Cassola, F. (2009). Studies of Tiger Beetles. CLXXVII. Notes on the tiger beetle fauna of Fiji (Coleoptera: Cicindelidae). Bishop Museum, 200927.
2) Pearson, D. L., & Cassola, F. (2005). A quantitative analysis of species descriptions of tiger beetles (Coleoptera: Cicindelidae), from 1758 to 2004, and notes about related developments in biodiversity studies. The Coleopterists Bulletin, 59(2), 184-193.
3) Putchkov, A. V., & Arndt, E. (1994). Preliminary list and key of known tiger beetle larvae (Coleoptera, Cicindelidae) of the world. Mitteilungen der Schweizerischen Entomologischen Gesellschaft, 67(3-4), 411-420.
4) Hill, J. M., & Knisley, C. B. (1992). Frugivory in the tiger beetle, Cicindela repanda (Coleoptera: Cicindelidae). The Coleopterists' Bulletin, 306-310.
5) Gilbert, C. (1997). Visual control of cursorial prey pursuit by tiger beetles (Cicindelidae). Journal of Comparative Physiology A, 181(3), 217-230.
6) Evans, M. E. G. (1965). The feeding method of Cicindela hybrida L.(Coleoptera: Cicindelidae). In Proceedings of the Royal Entomological Society of London. Series A, General Entomology (Vol. 40, No. 4‐6, pp. 61-66). Blackwell Publishing Ltd.
7) Swiecimski, J. (1957). The role of sight and memory in food capture by predatory beetles of the species Cicindela hybrida L.(Coleoptera: Cicindelidae).Polsk Pismo Entomol, 26, 205-232.
8) Pearson, D. L. (1988). Biology of tiger beetles. Annual Review of Entomology,33(1), 123-147.
9) Choate, P. M. (2008). Tiger Beetles (Coleoptera: Carabidae: Collyrinae and Cicindelinae). In Encyclopedia of Entomology (pp. 3804-3818). Springer Netherlands.
10) Freitag, R. (1974). Selection for a non-genitalic mating structure in female tiger beetles of the genus Cicindela (Coleoptera: Cicindelidae). The Canadian Entomologist, 106(06), 561-568.
11) Pearson, D. L., & Knisley, C. B. (1985). Evidence for food as a limiting resource in the life cycle of tiger beetles (Coleoptera: Cicindelidae). Oikos, 161-168.
12) Knisley, C. B., Reeves, D. L., & Stephens, G. T. (1989). Behavior and development of the wasp Pterombrus rufiventris hyalinatus Krombein (Hymenoptera: Tiphiidae), a parasite of larval tiger beetles (Coleoptera: Cicindelidae). Proceedings of the Entomological Society of Washington, 91.
13 Palmer, M. K. (1982). Biology and behavior of two species of Anthrax (Diptera: Bombyliidae), parasitoids of the larvae of tiger beetles (Coleoptera: Cicindelidae). Annals of the Entomological Society of America, 75(1), 61-70.
14) Schultz, T. D. (1986). Role of structural colors in predator avoidance by tiger beetles of the genus Cincindela (Coleoptera: Cincindelidae). Bulletin of the ESA, 32(3), 142-146.
15) Kamoun, S., & Hogenhout, S. A. (1996). Flightlessness and rapid terrestrial locomotion in tiger beetles of the Cicindela L. subgenus Rivacindela van Nidek from saline habitats of Australia (Coleoptera: Cicindelidae). The Coleopterists' Bulletin, 221-230.
16) Knisley, C. B., & Pearson, D. L. (1981). The function of turret building behaviour in the larval tiger beetle, Cicindela willistoni (Coleoptera: Cicindelidae). Ecological Entomology, 6(4), 401-410.
17) Zerm, M., & Adis, J. (2003). Exceptional anoxia resistance in larval tiger beetle, Phaeoxantha klugii (Coleoptera: Cicindelidae). Physiological Entomology, 28(2), 150-153.
18) Sastry, K. S. S., & Appanna, M. (1958). Parasites and predators of some of the common insect pests of sugarcane in Visvesvaraya Canal Tract, Mandya District, Mysore State. Mysore agric. J, 33, 140-149.
19) Macfie, J. W. S. (1923). A Note on a Beetle which preys on Mosquito Larvae.Bulletin of Entomological Research, 13(04), 403-403.
20) Nachappa, P., Braman, S. K., Guillebeau, L. P., & All, J. N. (2006). Functional response of the tiger beetle Megacephala carolina carolina (Coleoptera: Carabidae) on twolined spittlebug (Hemiptera: Cercopidae) and fall armyworm (Lepidoptera: Noctuidae). Journal of economic entomology, 99(5), 1583-1589.
21) Sinu, P. A., Nasser, M., & Rajan, P. D. (2006). Feeding Fauna and Foraging Habits of Tiger Beetles Found in Agro‐ecosystems in Western Ghats, India. Biotropica, 38(4), 500-507.
22) Knisley, C. B. (2011). Anthropogenic disturbances and rare tiger beetle habitats: benefits, risks, and implications for conservation. Terrestrial Arthropod Reviews, 4(1), 41-61.
23) Pearson, D. L., & Cassola, F. (1992). World‐wide species richness patterns of tiger beetles (Coleoptera: Cicindelidae): indicator taxon for biodiversity and conservation studies. Conservation Biology, 6(3), 376-391.
24) Rodríguez, J. P., Pearson, D. L., & Barrera, R. R. (1998). A test for the adequacy of bioindicator taxa: are tiger beetles (Coleoptera: Cicindelidae) appropriate indicators for monitoring the degradation of tropical forests in Venezuela?. Biological Conservation, 83(1), 69-76.
25) Aydın, G., Şekeroğlu, E., & Arndt, E. (2005). Tiger beetles as bioindicators of habitat degradation in the Çukurova Delta, southern Turkey. Zoology in the Middle East, 36(1), 51-58.
26) Pearson, D. L., & Cassola, F. (2007). Are we doomed to repeat history? A model of the past using tiger beetles (Coleoptera: Cicindelidae) and conservation biology to anticipate the future. In Beetle Conservation (pp. 47-59). Springer Netherlands.
27 Jardine, W. (1835). The Naturalist's Library, vol. i. p. 117.
28) Fabricius, J. C. (1801). Systema eleutheratorum secundum ordines, genera, species adiectis synonymis, locis, observationibus, descriptionibus.-Kiliae, Bibliopolium academ. novvm 1801-1802 (Vol. 2). Bibliopolium academ. novvm.
29) Pearson, D. L., & Vogler, A. P. (2001). Tiger beetles: the evolution, ecology, and diversity of the cicindelids. Cornell University Press.
30) Zimsen, E. (1964). The type material of IC Fabricius. Munksgaard, Copenhagen, Denmark. p. 64.
31) Hom, W. (1926). Pars 86: Carabidae: Cicindelinae. Coleopterorum Catalogus, 345. Berlin: Junk & Schenkling.
32) Maddison, D., Baker, M. D., & Ober, K. (1999). Phylogeny of carabid beetles as inferred from 18S ribosomal DNA (Coleoptera: Carabidae). Systematic Entomology, 24(2), 103-138.
33) Erwin, T. L. (1985). The taxon pulse: a general pattern of lineage radiation and extinction among carabid beetles. Taxonomy, phylogeny and biogeography of beetles and ants, 437-488.
34) Cassola, F. (2001). Studies of tiger beetles CXXIII Preliminary approach to the macrosystematics of the tiger beetles (Coleoptera: Cicindelidae). Russian Entomological Journal, 10, 265–272.
35) Liebherr, J. K., & Will, K. W. (1998). Inferring phylogenetic relationships within Carabidae (Insecta, Coleoptera) from characters of the female reproductive tract. Phylogeny and classification of Caraboidea (Coleoptera: Adephaga). Museo Regionale di Scienze Naturali, Torino, 107-170.
36) Erwin TL, Pearson DL (2008) A Treatise on the Western Hemisphere Caraboidea (Coleoptera), Their Classification, Distribution, and Ways of Life. Volume II (Carabidae-- Nebriiformes 2-- Cicindelitae). Pensoft, Sofia- Moscow, p. 18.
37) Rivalier, E. (1954). De´membrement du genre Cicindela Linne. II. Faune americaine. Rev. Fr. Entomol., 21, 249–268.
38) Vogler, A. P., & Welsh, A. (1997). Phylogeny of North American< i> Cicindela</i> Tiger Beetles Inferred from Multiple Mitochondrial DNA Sequences. Molecular phylogenetics and evolution, 8(2), 225-235.
39) Pons, J., Barraclough, T. G., Theodorides, K., Cardoso, A., & Vogler, A. P. (2004). Using exon and intron sequences of the gene Mp20 to resolve basal relationships in Cicindela (Coleoptera: Cicindelidae). Systematic biology, 53(4), 554-570.
40) Basford, N. L., Butler, J. E., Leone, C. A., & Rohlf, F. J. (1968). Immunologic comparisons of selected Coleoptera with analyses of relationships using numerical taxonomic methods. Systematic Biology, 17(4), 388-406.
41) Kuster, J. E. (1979). Comparative structure of compound eyes of Cicindelidae and Carabidae (Coleoptera): evolution of scotopy and photopy. Quaestiones Entomologicae, 15.
42) Shelford, V. E. (1917). Color and Color-pattern Mechanism of Tiger Beetles, with Twenty-nine Black and Three Colored Plates (Vol. 3, No. 4). University of Illinois.
43) Stegemann, F. (1930). Die Fliigeldecken der Cicindelinae. Ein Beitrag zur Kenntnis der Insektencuticula. Z. Morphol. Okol. Tiere, 18, 1-73.
44) Ward, R. D. (1979). Metathoracic wing structures as phylogenetic indicators in the Adephaga (Coleoptera). In Carabid Beetles (pp. 181-191). Springer Netherlands.
45) Regenfuss, H. (1975). Die Antennen Putzeinrichtung der Adephaga (Coleoptera), parallele evolutive Vervollkommnung einer komplexen Struktur. Z. Zool. Syst. Evolutionsforsch, 13, 278-99.
46) Ball, G. E., Acorn, J. H., & Shpeley, D. (2011). Mandibles and labrum-epipharynx of tiger beetles: basic structure and evolution (Coleoptera, Carabidae, Cicindelitae). ZooKeys, (147), 39.
47) Serrano, J., & Yadav, J. S. (1984). Chromosome numbers and sex-determining mechanisms in adephagan Coleoptera. The Coleopterists' Bulletin, 335-357.
48) Barraclough, T. G., & Vogler, A. P. (2002). Recent diversification rates in North American tiger beetles estimated from a dated mtDNA phylogenetic tree. Molecular Biology and Evolution, 19(10), 1706-1716.
49) Proença, S. J., Collares-Pereira, M. J., & Serrano, A. R. (2004). Cytogenetic variability in three species of the genus Cicindela (sl)(Coleoptera, Cicindelidae): Karyotypes and localization of 18S rDNA genes. Genetics and Molecular Biology, 27(4), 555-560.
50) Brust, M. L., Hoback, W. W., & Johnson, J. J. (2010). Fishing for tigers: A method for collecting tiger beetle larvae holds useful applications for biology and conservation. Coleopterists Bulletin, 64(4), 313-318.
Distribution References
Acciavatti, R. E., & Pearson, D. L. (1989). The tiger beetle genus Cicindela (Coleoptera, Insecta) from the Indian subcontinent (Vol. 58). Carnegie Museum of Natural History.
Cassola, F. (2006). Studies of tiger beetles. CLXIII. New records from Singapore (Coleoptera: Cicindelidae). Bulletin de l'Institut Royal des Sciences Naturelles de Belgique Entomologie, 76, 25-29.
Muhabbet, K. (2008). Cicindela (Cosmodela) aurulenta Fabr. in North Thailand (Coleoptera, Cicindelidae). CESA News, 36, 4-5.
Shigehisa, H. (2002). New record of Cicindela aurulenta Fabricius from Japan (Coleoptera, Cicindelidae). Gekkan-Mushi, 379, 14-15.
Acknowledgements
This webpage would not be possible without the following people:
Mr. Foo Maosheng, who helped me with catching the beetles and provided me with termites, containers and videos.
Dr. Alexey Solodonikov from the Zoological Museum - University of Copenhagen (ZMUC) for information on the type specimens.
Mr. Nicky Bay who kindly agreed to allow me to use his images from http://sgmacro.blogspot.sg.
Contact Me
Darren Yeo is contactable at cloudfire@gmail.com













